99m Tc-MIBI elimination by a tumor as predictor of
pathological effect of chemotherapy in locally
advanced breast cancer patients.
Portnoj S.M., Shiryaev S.V., Odjarova A.A., Anurova O.A., Laktionov K.P., Rjabchikov D.A.
N.N.Blokhin Russian Cancer Research Center, Moscow, Russia.
Introduction
Effective chemotherapy can convert locally advanced breast cancer to operable form and give chance for survival.
Ineffective induction therapy means incurability on the whole. Predicting of the chemotherapy effect may give possibility to
select optimal kind of therapy.
Aim
To estimate predictive significance of 99mTc-MIBI accumulation and elimination by tumor on the effectiveness of neoadjuvant
chemotherapy in locally advanced breast cancer patients.
Materials and methods
Investigation of accumulation and elimination of 99mTc-MIBI by tumor was performed in 99 breast cancer patients
(stages: IIa – 6, IIb – 9, IIIa – 13, IIIb – 56, IIIc – 15 patients) before the beginning of chemotherapy
(CAF, FAC, docetaxel, 3-6 cycles). 99mTc-MIBI was introduced intravenously (555 MBq), with the following
two-phase (in 15 min and in average 3 hours) static scintigraphy of a breast.
Relative accumulation (RA) of 99mTc-MIBI in tumors in 15 min after injection (RA1), RA after 3 hours (RA2), and percent of
elimination (PE) were calculated [PE=(RA1- RA2)x100/RA1]. 85 patients were operated and pathological effect can be
evaluated in these cases. “No residual tumor” and “Microscopic residual tumor”
were united as “pathological effect”.
Results
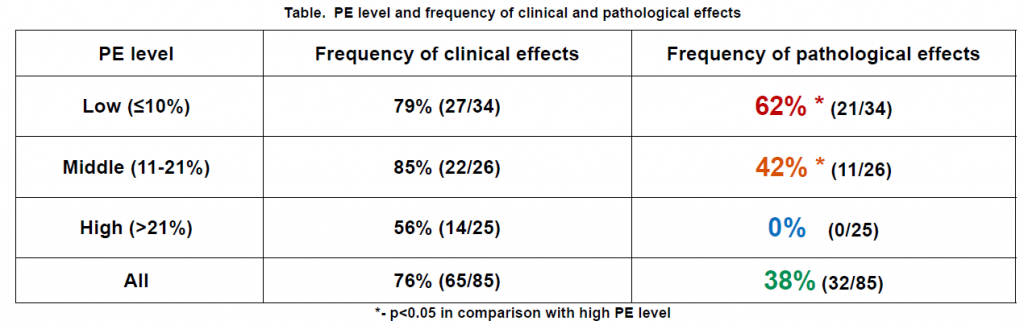
Clinical effect was observed in 76% (complete effect – in 11, partial effect – in 52, stabilization – in 21, and progression – in
1 patient). Pathological effect was observed in 38% (no residual tumor – in 14, and microscopic residual tumor – in 18
cases). In patients with high level of the PE pathological effect was not attained (see table). There were more strong
predicting pathological effect factors, calculated by regression analysis:
PE (p=0,00008), RA2 (p=0,003), Pgp-170 (p=0,023),
KI-67 (p=0,033), ER (p=0,067)
Discussion
The best characterized mechanism of multidrug resistance (MDR) in cancer involves the MDR1 efflux transporter Pglycoprotein.
The gamma-emitting organotechnetium complex, hexakis(2-methoxyisobutylisonitrile)technetium (99mTc-MIBI)
was validated as a substrate for MDR1 P-glycoprotein (1) and as a substrate for the multidrug resistance-associated protein
pump (2). Ciarmiello A, et al. demonstrated in locally advanced breast cancer patients, that a rapid tumor clearance of
99mTc-sestamibi may predict lack of tumor response to neoadjuvant chemotherapy. Del Vecchio S., et al. found that early
uptake of 99mTc-MIBI in breast carcinomas is affected by alterations of apoptotic pathway. High levels of Bcl-2 prevented
accumulation of 99mTc-MIBI in tumors (4). In this paper we reproduce results of the Ciarmiello A, et al. (3): 1/3 of breast
cancer patients had rapid 99mTc-MIBI elimination by a tumor, and such patients had not pathological response after induction
chemotherapy.
Conclusion
Our first results confirm the main hypothesis: rapid 99mTc- MIBI elimination by a tumor predicts the low pathological response
to chemotherapy. Detection of the high level of 99mTc-MIBI PE by tumor can indicate that neoadjuvant target or endocrine
therapy may be more preferential, than chemotherapy.
References
1. Piwnica-Worms D., Chiu M.L., Budding M., et al. Functional imaging of multidrug-resistant P-glycoprotein with an organotechnetium complex. //Cancer Res. 1993.-53.-5.- p.977-984.
2. Hendrikse N.H., Franssen E.J., van der Graaf W.T., et al. 99mTc-sestaMIBI is a substrate for P-glycoprotein and the multidrug resistance-associated protein. //Br J Cancer. 1998.-77.-3.-p.353-358.
3. Ciarmiello A., Del Vecchio S., Silvestro P., et al. Tumor clearance of technetium 99m-sestaMIBI as a predictor of response to neoadjuvant chemotherapy for locally advanced breast cancer. //J Clin Oncol. 1998.-16.-5.- p.1677-1683.
4. Del Vecchio S., Zannetti A., Aloj L., et al. Inhibition of early 99mTc-MIBI uptake by Bcl-2 anti-apoptotic protein overexpression in untreated breast carcinoma. // Eur J Nucl Med Mol Imaging. 2003.-30.-6.- p.879-887.